Staying Ahead of E-prescribing Requirements
Hit ENTER to search or ESC to close.
Staying Ahead of E-prescribing Requirements

If your platform isn’t compliant with the latest industry and regulatory certifications, your customers are at risk, putting your customer retention in jeopardy as well.

ONC HTI-1 Final Rule
The Office of the National Coordinator for Health Information Technology (ONC) has finalized new standards for health IT certification, including lengthy requirements for use of AI in healthcare workflows.
DrFirst’s clinical-grade AI has a 10-year track record of patient safety and clinician efficiency.

E-Prescribing Standard Upgrades
CMS has issued a proposed rule that would require adoption of new versions of standards for many core medication transactions, including: Electronic Prescribing, Medication History, Formulary & Benefits, Electronic Prior Authorization, and Real-Time Prescription Benefits.

EPCS and PDMP Mandates
DrFirst was the first company in the nation to have a certified EPCS solution and laid the groundwork for electronic prescribing of controlled substances. Since EPCS became legalized in all 50 states and the District of Columbia, a majority of states have gone on to mandate the use of EPCS for all controlled substances.
View Map
Since January 1, 2023, the Centers for Medicare & Medicaid Services (CMS) requires all controlled substance prescriptions under the Medicare Part D drug plan to be transmitted electronically.
With standards and regulatory requirements changing constantly, extensive legal, compliance, and product development resources are necessary to stay current. This burden challenges your roadmap commitments, competing priorities, and already scare resources.
If your platform isn’t compliant with the latest industry and regulatory certifications, your customers are at risk, putting your customer retention in jeopardy as well.

Cures Act Final Rule

State EPCS and PDMP Mandates
DrFirst has been at the forefront of electronic prescribing of controlled substances (EPCS) since it sent the nation’s first electronic prescription of a controlled substance. With EPCS legalized nationally, a majority of states have since mandated the use of EPCS for all controlled substances. View Map

Federal EPCS Compliance
The Centers for Medicare & Medicaid Services (CMS) delayed the mandate deadline to January 1, 2023, for the SUPPORT Act. This requires all controlled substance prescriptions under Medicare’s Part D drug plan to be transmitted electronically.
With standards and regulatory requirements changing constantly, extensive legal, compliance, and product development resources are necessary to stay current. This burden challenges your roadmap commitments, competing priorities, and already scare resources.
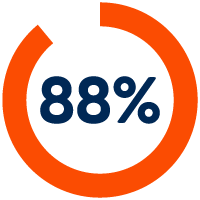
Partners who say our software integrations improved their regulatory compliance and quality measures2

Nick Barger, PharmD, Vice President, Product at DrFirst teams up with Healthcare IT Today to create the Healthcare Regulatory Talk Series to explore emerging trends in regulatory. Are you ready to navigate the regulatory landscape with confidence?
Get instant access to keep compliance on track with our curated collection of videos, blogs, and news articles on the latest e-prescribing updates.


Outsource your regulatory challenges by integrating DrFirst’s solutions seamlessly with existing workflows to:
1. Office of the National Coordinator for Health Information Technology
2. 2021 Techvalidate survey