Integrated workflows ease staff burden and satisfy regulatory mandates.

Welcome to the Regulatory Hub
Breaking News
The HTI-4 Final Rule, adopted July 31, 2025, updates the ASTP/ONC Health IT Certification Program with new standards for electronic prescribing, real-time prescription benefit checks, and electronic prior authorization to reduce administrative burden and improve healthcare efficiency.
Key compliance deadlines:- January 1, 2027—mandatory inclusion of electronic prior authorization and real-time prescription benefit capabilities in the Base EHR definition.
- January 1, 2028—upgrade to NCPDP SCRIPT Standard version 2023011.
The rule requires a large overhaul of e-prescribing, while simultaneously expanding interoperability and prior authorization systems that support patient care and public health goals. It includes five new certification criteria, six standards updates, and nine revised certification criteria that will impact roadmap development in 2025, 2026, and 2027.
HTI-2 Top Takeaways:
Total E-Prescribing Upgrade Required: Expands CMS’ recently finalized e-prescribing standard, requiring the adoption of NCPDP SCRIPT Standard 2023011, Formulary and Benefit Version 60, and electronic prior authorization.
Streamlined Prior Authorizations: Mandates the integration of standardized APIs for medical prior authorization to improve data exchange between health IT systems and payers, and to reduce delays and administrative burdens for patients and providers.
Enhanced Real-Time Price Transparency: Requires health IT systems to include functionalities that allow for immediate access to drug pricing information for patients.
Improved Data Interoperability: Mandates standardized APIs across different health IT systems, promoting better coordination and continuity of care.
On June 13, 2024, the Centers for Medicare & Medicaid Services (CMS) and the Office of the National Coordinator for Health Information Technology (ONC) issued a final rule revising standards for electronic prescribing, prior authorization, and real-time benefits under Medicare Part D.
Some top takeaways:
- Formulary and benefit version 60 is required by January 1, 2027
- Real-Time Prescription Benefit version 13 is required by January 1, 2027
- All e-prescribing routing transactions are required to use NCPDP SCRIPT version 2023011 by January 1, 2028
- Electronic Prior Authorization is upgraded to NCPDP SCRIPT 2023011 by January 1, 2028
- Medication History is upgraded to NCPDP SCRIPT 2023011 by January 1, 2028
View the full details here.
Find regulatory resources of all kinds to help you stay ahead.
Explore the topics you care about and find expert resources to help you succeed.

After cruising for the first half of the year, new regulations are shifting things into high gear with a final rule that makes electronic health record (EHR) vendors responsible for integrating real-time prescription benefit (RTPB) checks and electronic prior authorization (ePA) directly into physician workflows.
Published by ![]()
Topic: Prior Authorization
Read More

Healthcare regulations are shifting, but the deadlines are still the deadlines. According to Nick Barger, PharmD, VP of Product at DrFirst, uncertainty around the HHS and CMS doesn’t mean compliance can fall by the wayside.
Published by ![]()
Topic: The Centers for Medicare & Medicaid Services (CMS)
Read More

Is your organization equipped to meet the fast-approaching regulatory deadlines? Get up to speed on HTI-1, CMS-0057-F, and HTI-2 requirements so you can determine the fastest route to the compliance finish line before the deadlines.
Topics: The Center for Medicare & Medicaid Services (CMS)
Download Now
Prior authorization (PA) is the poster child for administrative tasks that spawn inefficiencies in our healthcare system, impacting providers and patients alike.
Published by ![]()
Topics: Prior Authorization
Read More
As an EHR or HIT vendor, navigating the regulatory landscape can feel like you’re traversing unfamiliar roads with signposts for compliance around every turn. Our Regulatory Roadmap is here to help.
Topics: E-Prescribing
Download Now
With the public comment period now closed, the newly renamed Assistant Secretary for Technology Policy and Office of the National Coordinator for Health Information Technology (ASTP/ONC) has received substantial feedback...
Published by ![]()
Topics: E-Prescribing | Price Transparency

Join us for a webinar that will focus on the key policy updates introduced by HTI-2, including the required upgrades to NCPDP SCRIPT version 2023011 and the mandatory support for Electronic Prior Authorization (ePA), Structured and Codified Sig, and Real-Time Prescription Benefit (RTPB).
Topics: HTI-2 Compliance | ONC | ASTP
Watch Now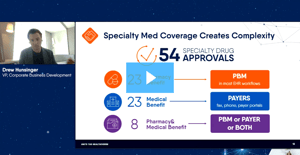
In this webinar, DrFirst specialty medication access experts Drew Hunsinger and Tyler Wince explore critical issues shaping the specialty medication market, delve into the complexities of healthcare access, and discuss proactive solutions that transcend regulatory requirements.
Topics: Specialty Medication | Prior Authorization
Watch Now
Rules from CMS and ASTP/ONC raise the bar for e-prescribing and interoperability, yielding challenges and opportunities for EHR and HIT vendors.
Topics: E-Prescribing
Download Now
In this far-ranging discussion, two experts in health care explore current and potential solutions to the much-hated process of prior authorization in medical care.
Published by ![]()
Topics: Prior Authorization

The new final rule issued by CMS on June 13, 2024, goes beyond a basic upgrade of SCRIPT standards and improves care connections between doctors, pharmacies, and patients.
Topic: The Centers for Medicare & Medicaid Services (CMS)
Watch now
Before the ONC newly proposed HTI-2 rule, announced on July 10, becomes a primary focus for health IT developers, let’s explore what EHRs need to understand and implement to comply with CMS’ final rule for e-prescribing.
Published by ![]()
Topic: The Centers for Medicare & Medicaid Services (CMS)
Read More
Learn about new rules on the horizon from The Centers for Medicare & Medicaid Services (CMS) and the impact they will have on your organization and development roadmap.
Topic: The Centers for Medicare & Medicaid Services (CMS)
Watch Now
E-prescribing of controlled substances (EPCS) has grown rapidly over the past ten years, not only because technology has improved but also due to the substantial benefits for patients and physicians.
Topics: E-Prescribing | E-Prescribing of Controlled Substances (EPCS)

In a discussion with athenahealth's CMO Nele Jessel, M.D., and John Lynn from Healthcare IT Today, our CEO Cam Deemer delved into the evolution of e-prescribing and necessary improvements for prescribers and patients.
Topics: E-Prescribing | Medication Management
Colin Banas and Nick Barger will help you get moving on improvements in information sharing, new guardrails for AI-enabled tools, patient data access, and a new certification process.
Topics: The Office of the National Coordinator (ONC) | The Centers for Medicare & Medicaid Services (CMS)
Watch Now
On Healthcare IT Today, Cam Deemer and Pooja Babbrah delved into pharmacy data accessibility with John Lynn.
Topic: The Office of the National Coordinator (ONC)
Read More
You might have heard that the Office of the National Coordinator (ONC) for Health Information Technology recently released a proposed rule aimed at...
Topic: The Office of the National Coordinator (ONC)

For EHR and healthcare technology systems vendors, recent proposals reveal important changes that will impact your development roadmap. The best time to get ready? Right now.
Topics: Medication Management | E-Prescribing

In the rapidly evolving regulatory landscape, EHR vendors must keep abreast of state and federal deadlines to maintain provider compliance and prevent adverse effects on patient care. Stay informed with the latest mandates, standards, and legislation.
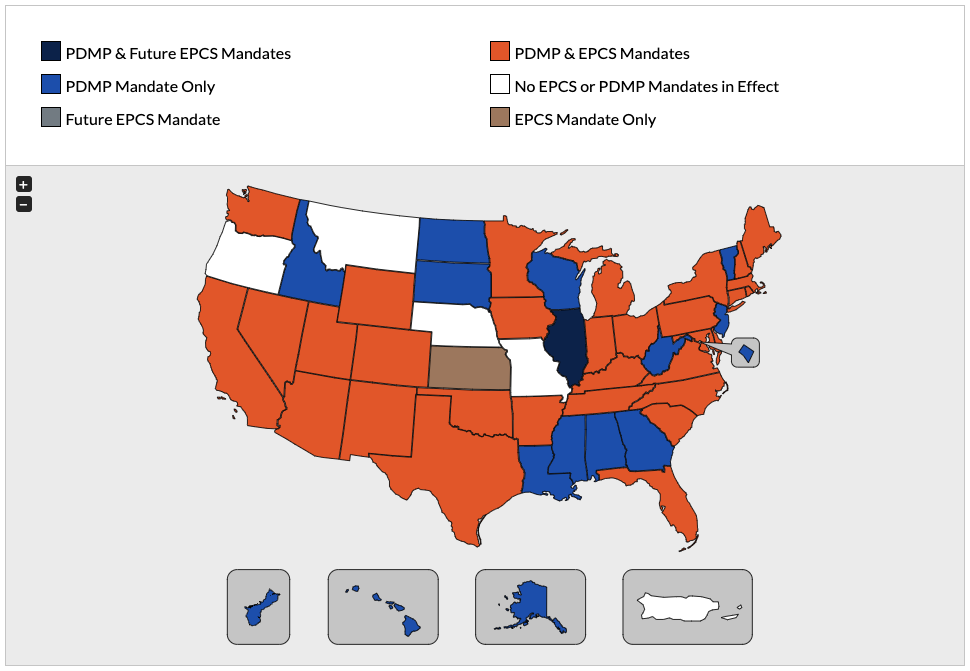
Topics: E-Prescribing | E-Prescribing of Controlled Substances (EPCS) | Prescription Drug Monitoring Programs (PDMP) | The Centers for Medicare & Medicaid Services (CMS)

PDMPs, or prescription drug monitoring programs (sometimes shortened to PMP), were created by individual state legislation years ago to reduce prescription drug abuse and diversion.
Topic: Prescription Drug Monitoring Programs (PDMP)

Last December's data standards from the ONC mark a major expansion in health IT regulations, potentially disrupting product plans for years.
Published by ![]()
Topic: The Office of the National Coordinator (ONC)

There’s a collective holding of breath right now in health IT as the ONC appears likely to issue its final HTI-1 rule with new data standards for the next stage of healthcare interoperability soon.
Published by ![]()
Topic: The Office of the National Coordinator (ONC)

More regulatory churn is coming to shake up e-prescribing, this time with new data standards to step up clinical decision support at the point of care.
Published by ![]()
Topic: The Centers for Medicare & Medicaid Services (CMS)
John Lynn of Healthcare IT Today discusses e-prescribing's evolution and pharmacy interoperability challenges with DrFirst CEO Cam Deemer and Point of Care Partners' Pooja Babbrah.
Published by ![]()
Topic: The Office of the National Coordinator (ONC)
Watch Now
While e-prescribing is one of the biggest healthcare interoperability successes, there is still plenty of work to be done to improve how medication is managed.
Published by ![]()
Topics: Medication Management | E-Prescribing

Grace Cordovano, PhD, and DrFirst's CMO Colin Banas discuss how new data sharing rules improve transparency for clinicians and patients, especially during prior authorization.
Topics: Medication Management | Prior Authorization
Watch NowFederal EPCS Requirement
As of January 1, 2023, The Centers for Medicare & Medicaid Services (CMS) requires all controlled substance prescriptions under Medicare’s Part D drug plan to be transmitted electronically. Additionally, the new compliance date for electronic prescriptions in Long Term Care facilities is January 1, 2025.
Click the map below to discover EPCS & PDMP compliance state requirements.